The primary emphasis of the Hegde lab’s research is genome damage, repair, inhibitions/deficiencies in neurons, and exploring the DNA damage response (DDR) targeted approaches involved in preventing neuronal dysfunction during aging and brain disorders.
Key discoveries in the last five years from the Hegde lab
- We were the first to demonstrate that TDP-43/FUS implicated in motor neuron diseases are essential for DNA strand break repair in neurons (published in Nature Communications, PNAS, and Hum Mol Genet; highlighted in various national/international media and Faculty1000). Received best peer-reviewed publication award.
- We discovered a new mechanism of DNA damage signaling in stroke and therapeutic strategy.
- We discovered that alpha-synuclein toxicity in Parkinson’s disease impairs mitochondrial import of DNA repair and antioxidant machinery in mitochondria by Parkin-mediated degradation of Tom40.
- We discovered novel neurodegenerative etiologies, including pro-oxidant metals inhibit DNA repair enzymes by their oxidizing cysteine residues. Thus, reversal of such effect requires a combination of metal chelation and cysteine reduction (published in JBC and JAD). This work has been highlighted in local and international media. In recognition of this work, the journal, Chemical Research in Toxicology highlighted these findings in its spotlight section.
- We were the first to show the existence of an alternative end joining (Alt-EJ) mode of DNA double strand break repair in post-mitotic neurons.
- We have established hNSC and iPSC-derived primary neurons, inducible CRISPR based knockout/knock in strategy, unique amyotrophic lateral sclerosis (ALS) mouse models (generated by us), and state-of-the-art molecular and cellular biology approaches for analyzing DNA damage/repair parameters.
These findings have set the stage for DNA repair-based therapeutics aimed at preventing neurodegeneration and stroke.
Major Ongoing Projects
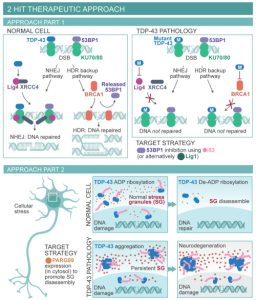
Novel role of TDP-43 in DNA damage response Implications and potential as a therapeutic target for TDP-43-associated neurodegeneration.
Genome damage and defective repair are etiologically linked to neurodegenerative disease. However, the underlying mechanisms remain enigmatic, which is a roadblock for developing effective therapy. We recently reported for the first time that TDP-43 participated in the DNA damage response in neurons and that its nuclear clearance in spinal motor neurons caused DNA double-strand break (DSB) repair defects in ALS. We documented that TDP-43 was a key component of the on-homologous end joining pathway of DSB repair, which was likely the major pathway for repair of DSBs in post-mitotic neurons. Our findings have uncovered a link between TDP-43 pathology and impaired DNA repair and suggest potential possibilities for DNA repair-targeted therapies for TDP-43-ALS, which are currently being explored in the Hegde Lab. These studies are supported by the National Institutes of Health (NIH) R01NS088645 (2015-2021) and R03AG064266 awards (2020-2022).
Molecular mechanisms of DNA repair defects in FUS-associated neurodegeneration
We have been working on defining the molecular basis of the role of RNA/DNA binding family proteins in genome maintenance and their implications in brain disorders since 2010. We have identified how hnRNP-U and other members of this family play specific roles in oxidative as well as DNA strand break repair. Our recent studies revealed defects in DNA nick ligation and oxidative damage repair caused by mutations in the RNA/DNA-binding protein FUS in familial ALS and fronto temporal dementia (FTD) patients. In healthy neurons, FUS protects the genome by facilitating PARP1-dependent recruitment of XRCC1/DNA Ligase IIIα (LigIII) to oxidized genome sites and activating LigIII via direct interaction. This is a critical step in the repair of oxidative genome damage, a foremost challenge for postmitotic neurons due to their high oxygen consumption. We discovered that mutant FUS significantly inhibited the recruitment of XRCC1/LigIII to DNA strand breaks, causing defects in DNA ligation during the repair of oxidative DNA damage, which contributed to neurodegeneration. These studies are the first to report a specific DNA repair defect. Further, they highlight the potential for DNA ligase-targeted approaches to slow down ALS/FTD. Our current studies aim define the mechanisms of the FUS-DNA repair impact the nuclear and mitochondrial genomes of ALS and FTD affected neurons and astrocytes and explore Ligase and/or PARP-1 as therapeutic targets. These studies are supported by our NIH RF1 NS112719 award (2020-2025).
Understanding the effect of hemin and iron in neuronal genome instability in hemorrhagic stroke and its prevention by a combinatorial nanozyme of antioxidant plus metal chelator
Dyshomeostasis in metal ions has been implicated in neurological diseases and stroke; however, metal-targeted therapies have not been as successful, as expected. We have made the following seminal discoveries: (a) Increase in one metal (as charged ions in cells) leads to a drastic change in the homeostasis of many metals and, thus, the effect of increase in a metal is not restricted to that metal alone; (b) Metal ions act as double-edged swords by not only inducing genome damage, but also inhibiting various DNA repair processes. This involves metal-mediated oxidative inhibition of these repair proteins, which is reversible. These initial discoveries led to a recent study from the Hegde laboratory in collaboration with Prof. Thomas Kent (Texas A&M University) and Prof. James Tour (Rice University) wherein they identified that Hemin/hemorrhage induces DNA DSBs in neurons, like a radiomimetic bleomycin leading to senescence and ferroptosis, which is amenable to a combinatorial nanozyme with anti-oxidant and iron-chelation activities. These studies are supported by our NIH R01NS094535 award (2020-2025).
Funding
External Grants: Dr. Hegde is currently the principal investigator (PI) on five grants; four grants are from the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Aging (NIA) of the NIH (R01NS088645, R01NS094535, multi-year R01 RF1 NS112719 and R03AG064266), and one is a sponsored research grant from the drug discovery company BridgeBio totaling ~$1M support per year. The total amount of external grants awarded to the Hegde Lab since 2014 is approximately $5.0M. In the last five years, Dr. Hegde has been awarded multiple grants from the NIH as well as other external research foundations, such as Muscular Dystrophy Association, Alzheimer’s Association, American Parkinson’s Disease Association, ALS Association, BridgeBio Industry, and Melo Foundation. Dr. Hegde has established productive collaborations within the Houston Methodist Research Institute, throughout the Texas Medical Center (e.g. Texas A&M, Rice University), across the United States (e.g. University of Texas Medical Branch at Galveston, University of Washington at Seattle, Binghamton University, New York), and internationally (e.g. Latin America [INDICASAT, Panama], Belgium [University of Belgium], Egypt [Alexandria University], and India [JSS University, Mysore, IISc, Bangalore, Mangalore University, Mangalore]).
1- Houston Methodist Academic Institute (HMAI) Laboratory Operating Fund
PI: Hegde ML (2013-2026)
The HMRI supports the overall goal of the Hegde lab and the Division of DNA Repair Research to delineate the basic mechanism and clinical implications of genome instability and damage responses in human brain diseases.
2- NIH/NINDS R01NS088645-01
PI: Hegde ML (2015-2022)
Etiological Linkage of DNA Damage/Repair Deficiency in Neurodegenerative Diseases.
The goal of this study is to characterize the role of TDP-43 in DNA repair and implications to amyotrophic lateral sclerosis.
3- NIH/NIA R03AG064266
PI: Hegde ML (2020-2023)
A New Conditional TDPΔNLS Knock-In Mouse Model Generated Using CRISPR/Cas9 Technology to Study the Linkage of TDP-43 Pathology to Motor and Cognitive Defects in ALS, FTD and ADRD.
The goal of this study is to generate and characterize a novel knock-in mouse model for TDP-43-ALS.
4- NIH/NINDS-NIA RF1NS112719
PI: Hegde ML (2020-2025)
Defining the Altered FUS-PARP1-DNA Ligase III Axis and Its Implications to Nuclear and Mitochondrial Genome Damage Response in Motor Neuron Disease.
The goal of this study is to investigate the interaction of FUS, PARP-1 and DNA Ligase III in central nervous system and implications to FUS-associated neurodegeneration.
5- NIH/NINDS R01NS094535
Multiple PIs: Hegde ML Kent TA, Tour, Zhao (2020-2025)
Novel Carbon Nanozyme Mechanisms for Traumatic Brain Injury.
The goal of this study is to define mechanisms of hemolyzed blood induced genome injury post TBI.
6- BridgeBio Drug Discovery Sponsored Research Grant (2021-22)
Completed Research Support (Last 5 Years)
NIH/NCI R01CA158910 Mitra (PI), Hegde ML (Co-I) 03/2012 – 12/2019
Muscular Dystrophy Association (MDA294842) Hegde ML (PI) 05/2014 – 03/2017
ALS Association (3113) Hegde ML (PI) 08/2014 – 06/2016
Parkinson’s Association Hegde ML (PI) 06/2008-
